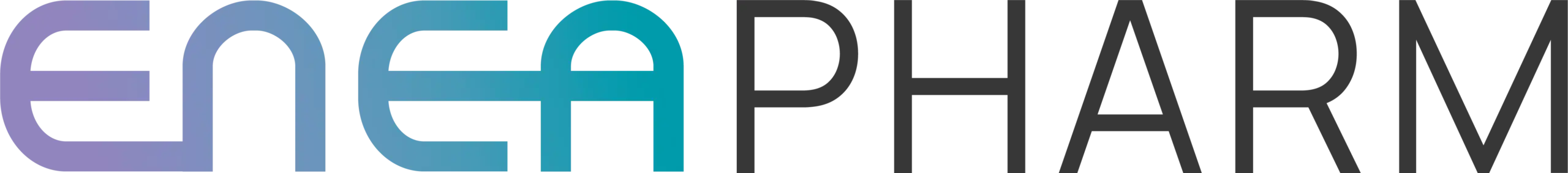Pipeline
Eneapharm is leveraging a robust and differentiated pipeline that encompasses a range of innovative delivery formats for enzymatic exocrine enzymes, currently progressing through various stages of development.
Among these, two candidates, EN3102 and EN3103 are specifically designed to address clearly identified unmet medical needs, as acknowledged by regulatory and health authorities. These therapeutic gaps remain unaddressed by existing treatments, highlighting a significant opportunity for differentiation. The absence of effective solutions in this segment not only underscores the medical relevance of Eneapharm’s approach but also represents a compelling economic and strategic opportunity in an underserved and high-potential market.
EN-311
Indication EPI : Adult
1
2
Research
1
2
Preclinical
1
2
2
2
Phase I / II
1
2
Phase III
1
2
Registration
EN-3102
Indication EPI : Paediatric
1
2
Research
1
2
Preclinical
1
2
Phase I
1
2
Phase II
1
2
Phase III
1
2
Registration
EN-3103
Indication EPI : Naso-gastric – First in class
1
2
Research
1
2
Preclinical
1
2
Phase I
1
2
Phase II
1
2
Phase III
1
2
Registration
EN-3104
Indication EPI : Improvment bioavailability
1
2
Research
1
2
Preclinical
1
2
Phase I
1
2
Phase II
1
2
Phase III
1
2
Registration
Adult form: capsule, stick, orodispersible tablet form.
Paediatric form: syrups, stick to be dispersed in any water phase taken by the child, water, fruit juices…